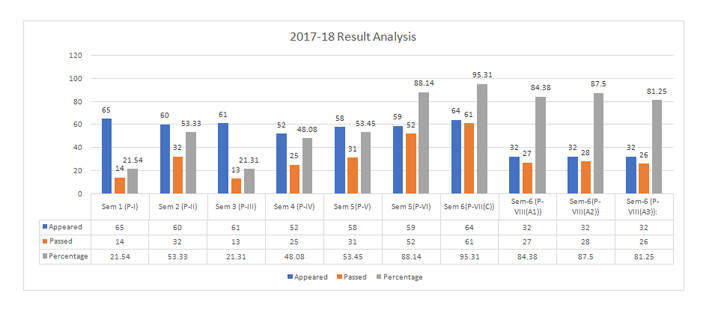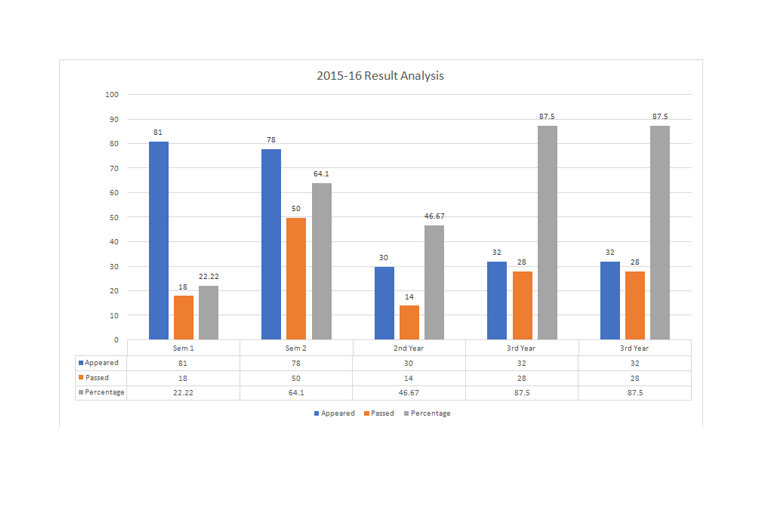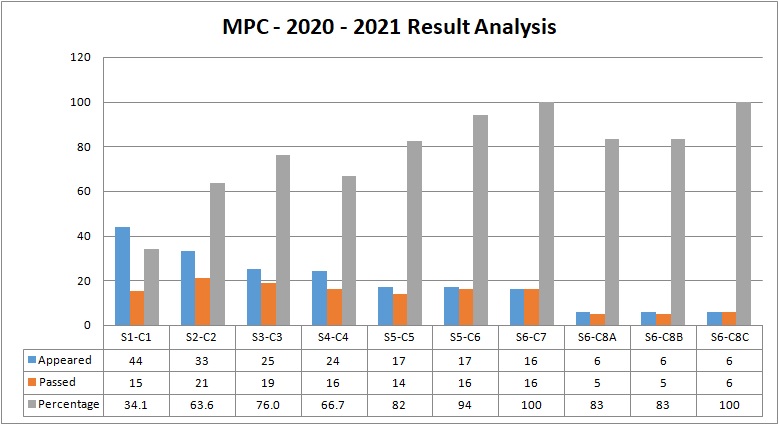
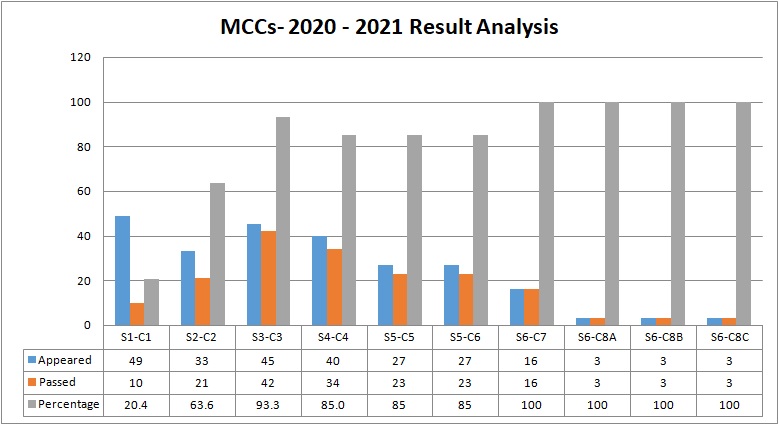
The Department of Chemistry came to existence in the year 1995. It offers four Under Graduate programs in Chemistry with the following combinations
MPC ( Mathematics, Physics, Chemistry)
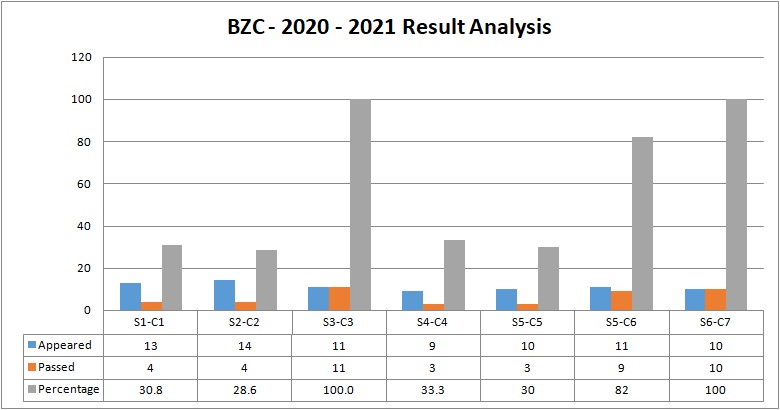
BZC (Botany, Zoology, Chemistry)
MCCs (Mathematics, Chemistry, Computer Science)
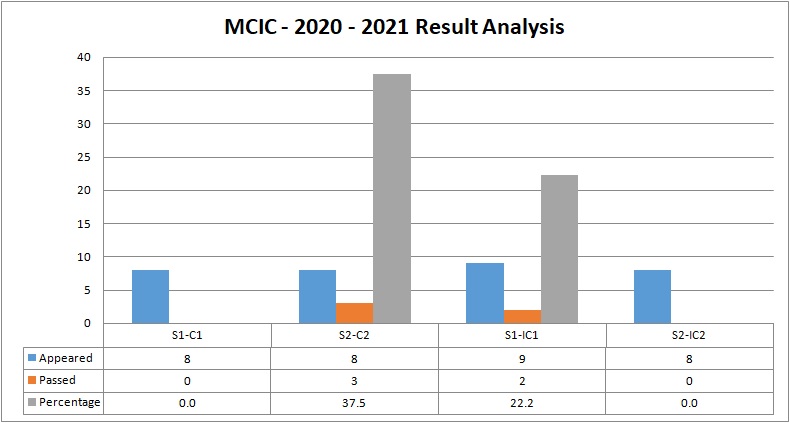
MCIC (Mathematics, Chemistry, Industrial Chemistry)
Many efficient teachers like Dr V.Venkateswarlu, Sri P. Rahmattullah Khan, Sri T.Sambasiva Rao, Dr B. Sateesh Kumar, Sri K.C.Vikram worked in the department. The Department of Chemistry is the biggest department in the college with four sanctioned posts and laboratories with adequate facilities. All the four sanctioned posts are filled with the regular faculty. The faculty of the department always strives hard to enrich the students’ knowledge and skills to suit the rapidly changing needs and aspirations of the society. The Department of Chemistry has always focused on the all round development of the students. They do their best effort to better the standards of the students. Besides academic activity faculty of the department also pursuing their research work and publishing papers.
S.No |
Name |
Designation |
Qualification |
Photo |
Curricullum Vitae |
| 01 | Sri. R.Yesupadamu | Assistant Professor | M.Sc. |  |
View CV |
| 02 | Sri. M Narasaiah | Assistant Professor | M.Sc. |  |
View CV |
| 03 | Sri.A.Lakshmana Rao | Assistant Professor | M.Sc. |  |
View CV |
| 04 | Ms M. Padmaja | Assistant Professor | M.Sc., M.Phil |  |
View CV |
S.No |
Name |
Designation |
Qualification |
|---|---|---|---|
| 01 | S. Gnana Kumari | Record Assistant | Intermediate |
S.No |
Year of Starting |
Title of the Program |
LEVEL (UG, PG) |
Duration (Years) |
Sanctioned Annual Intake |
1 |
1995 |
B.Sc (Mathematics, Physics, Chemistry) |
UG |
3 |
50 |
2 |
2015 |
B.Sc (Mathematics, Chemistry, Computer Science) |
UG |
3 |
60 |
3 |
2015 |
B.Sc (Botany, zoology, Chemistry) |
UG |
3 |
30 |
4 |
2020 |
B.Sc (Mathematics, Chemistry, Industrial Chemistry) |
UG |
3 |
30 |
Chemistry I SEM Inorganic & Organic Chemistry
1 After the completion of paper-I students are familiar with how properties of elements change in groups from top to bottom in p-block elements. they will clearly understand about different types of oxides.
2 They will know similarities between halogens and pseudo halogens. They are able to understand about organometallic compounds and difference between organic compounds and organic compounds.
3 Students will understand about different types of organic reactions and organic reagents.
4 After the completion of paper-I practicals students will be familiar with the procedure to identify anion and cation from the given salt.
Chemistry II SEM Physical&General Chemistry.
1 At the end of the Paper-II students are able to understand about symmetry of crystals, laws of crystals and x-ray diffraction of the crystals.
2 They understand about mesomorphic state and properties of liquid crystals.
3 Students clearly understand about critical phenomenon and Joule Thomson effect.
4 Students know about ideal solutions and non ideal solutions and Raoult’s law. They understand distillation, steam distillation, fractional distillation and importance of these techniques in the purification of compounds.
5 After the completion of the surface chemistry, students understand about importance of colloids in our daily life and uses of adsorption in chemistry reactions. Students will understand dipole moment , importance of dipole moment in understanding the structures of compounds and importance of molecular orbital theory over valency bond theory and hybridization theory.
6 Students understand about optical isomerism, planepolarized light ,enantiomers, diasteriomers, racemic mixture, resolution of racemic mixture, absolute configuration which are very important fundamental concepts.
7 At the end of semester-2 practicals students will be familiar with identification of two anions and two cations from the salt mixture and will know how to separate precipitates by using centrifuge.
Chemistry III SEM Inorganic & Organic Chemistry.
1 Students are familiarized with the Characteristic properties of d-block elements.
2 They clearly understand the different properties of metals according to valence bond theory, free electron theory and band theory.
3 They understand the stability of complexes according to EAN rule.
4 Students are able to understand different properties of f-block elements based on lanthanide contraction and actinide contraction.
5 They are familiar with the nomenclature of organic compounds with different functional groups.
6 Students clearly understood important named reactions and properties of organic compounds with different functional groups.
7 Students get good practical knowledge about standard solution and volumetric titrations.
8 They will be familiarized with identification of functional groups of organic compounds practically.
Chemistry IV SEM Spectroscopy and Physical Chemistry.
1 Students clearly understand Beer Lambert’s law which is essential for understanding Spectroscopy.
2 They wil be familiar about the concepts of different types of spectroscopic techniques and selection rules.
3 Students are able to understand colligative properties and the importance of colligative properties.
4 They will be familiar with kohlrauch’law and Debye Huckel’s Onsager ‘s equation and the application of conductivity measurements.
5 They understand about EMF, standard electrodes, applications of EMF measurements.
6 They can apply Phase rule and its applications in daily life.
7 Students get practical knowledge about critical solution temperature for different systems.
8 They got the practical knowledge of conductometric titrations.
Chemistry V SEM Inorganic, physical &Organic Chemistry.
1 Students understand different theories of coordination compounds and isomerism of complex compounds.
2 Gain knowledge about the properties of stability of complex compounds.
3 They will be able to write names of different nitrogen compounds according to IUPAC nomenclature.
4 Get familiar with important named reactions in nitrogen compounds.
5 Students will gain knowledge about themodynamic terms and laws.
6 Get experimental knowledge in the analysis of functional group of organic compounds.
Chemistry V SEM Inorganic, physical &Organic Chemistry.
1 Students understand reaction mechanisms of complex compounds.
2 Gain knowledge about the significance essential metals in our body and metalloporphyrins.
3 Students will be able to find rate of the reaction of different reactions and able to find order of the reactions.
4 Students will understand photochemical reactions and laws involved in it. They also understand the importance of photochemical reactions over thermal reactions.
5 They will be able to write structures of carbohydrates and amino acids. They understand the importance of amino acids and carbohydrates in our diet.
6 Students get the experimental knowledge in finding the rate of organic reactions. And they will be familiar with determination of surface tension and viscosity of liquids.
Chemistry VI SEM Environmental Chemistry
1 Students will gain knowledge about the concept of environmental chemistry terms and involved in it.
2 They will understand causes of air pollution, acid rains, green house effect and depletion of Ozone layer.
3 They understand causes of water pollution and criteria for water quality.
4 Students will be familiar with toxic chemicals in the environment and their effects.
5 They will be able to understand biodiversity and and its importance in ecosystem.
6 Water quality parameters can be known and students learn about the determination of hardness of water.
Chemistry VI SEM Organic spectroscopic techniques.
1 Students understand principles involved in Nuclear magnetic resonance spectroscopy and different processes involved in it.
2 They will understand electronic spectroscopy and the radiations involved in it. They gain knowledge about Franck-Condon principle and Born oppenheimer approximation.
3 They will gain knowledge about the chemical analysis by electronic sprectoscopy.
4 They understand basic principles in electon spin resonance spectroscopy and able to detect free radicals and ions in the spectra.
5 They Can prepare some organic compounds like aspirin, paracetamol and determine their yields.
Chemistry VI SEM Advanced organic reactions.
1 Able to understand basic principles involved in organic photochemistry and jablonsky diagram.
2 They will understand mechanisms involved in Norrish typeI and Norrish typeII cleavages.
3 Students understand protection of functional groups in organic reactions.
4 They will be familiar with new reactions and catalysts in organic chemistry like Suzuki coupling,Heck reaction.
5 They practically know about green procedures for chemical reactions like Diels-Alder reaction.
Chemistry VI SEM Pharmaceutical and Medicinal chemistry.
1 Students understand terminology in pharmaceutical chemistry and nomenclature of drugs.
2 Gain knowledge about generic drugs and non generic drugs their prices.
3 They gain knowledge about the classification of drugs and therapeutical activity of the drugs.
4 They will be familiar with immunity and white blood cells (CD4,CD8) which are involved in it.
5 Understand retrovirus and retroviral drugs to be used for controlling AIDS disease.
6 As a part of project work they get experimental knowledge about the hardness of water ,TDS of water and will be familiar with them.
PSO1: After completion of degree course, students are imparted with the theoretical knowledge and practical knowledge of handling chemicals and doing experiments.
PSO2: They are now eligible to enter into government sector related to chemistry particularly in food safety department, water testing laboratories and pharmacy department as pharmacist.
PSO3: In private sector, they can enter into pharmaceutical industries, paint industries and plastic industries as trainee chemist. They can also join into teaching field as chemistry teacher for high school students.
PSO4: These students are now eligible to study masters in chemistry in different fields of chemistry like Organic chemistry, Inorganic chemistry, Physical chemistry, Pharmaceutical chemistry,Medicinal chemistry, Industrial chemistry, Analytical chemistry etc.
| Seminars |  |
Quizzes |  |
|
| Assignments |  |
Remedial Classes |  |
|
| Group Discussions |  |
| Assignments |  |
Seminars |  |
|
| Group Discussions |  |
Remedial Classes |  |
|
| Quizzes |  |
| S.No | Date | Conducted By | Name of The Activity | Details of Resource Person | No of Participants | Report |
|---|---|---|---|---|---|---|
| 01 | 10-11-2021 | Chemistry & IQAC | World Science Day | Science Faculty | Click Here | |
| 02 | 02-12-2021 | Chemistry | National Pollution Control Day | Chemistry Faculty | Click Here | |
| 03 | 23-02-2022 to 08-04-2022 |
Chemistry | Certificate Course on "Role of Chemistry in Every Day Life" |
Chemistry Faculty | 30 | Click Here |
| Remedial Classes |  |
Seminars |  |
| Remedial Classes |  |
Seminars |  |
| Remedial Classes |  |
Seminars |  |
| Remedial Classes |  |
Seminars |  |